Welcome to ‘Walking the Curve’. We are so excited to share our enthusiasm about the great profession and craft of roasting coffee. We hope you love it as much as we do. Enjoy the second episode of ‘Behind the Roast’ with Willem Boot! Read the summary below.
The chemistry of coffee roasting has always fascinated me. We should consider the roasting process as a fundamental stage in a coffee’s journey from seed to cup. It unlocks the potential of the green coffee beans by creating hundreds of aromatic compounds. It also makes the beans brittle and porous enough to prepare the coffee for grinding and brewing.
Drying Phase
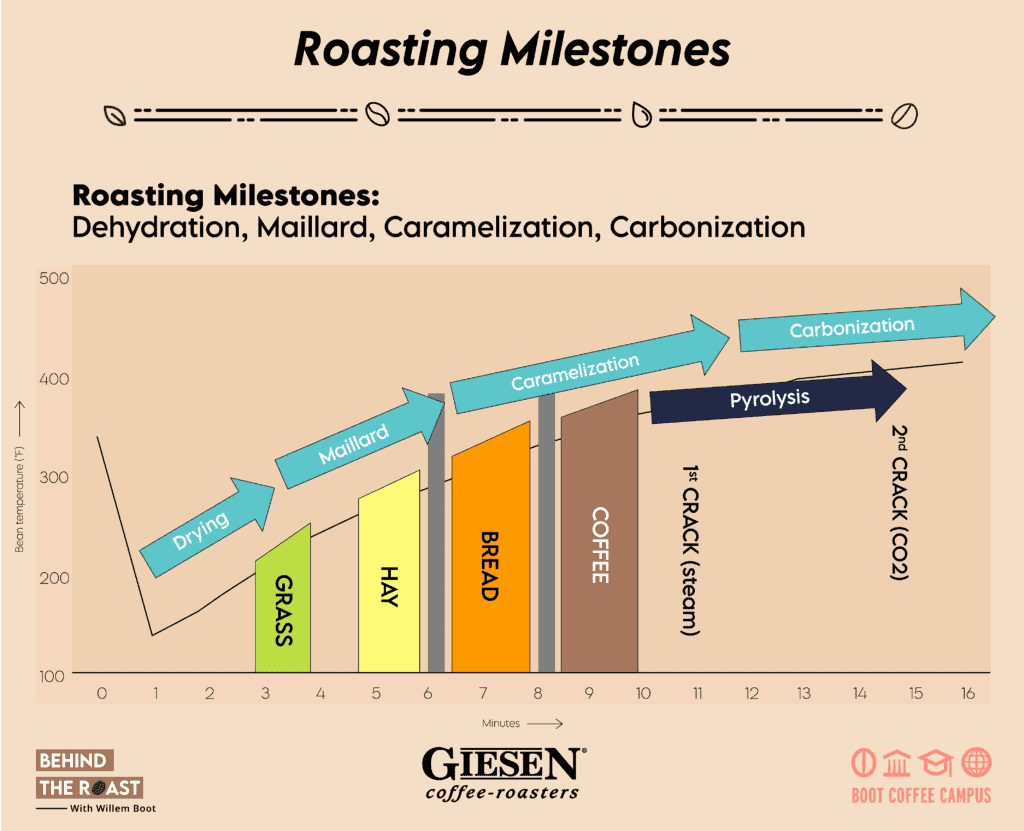
First, there is the Drying Phase. As soon as we charge the green beans into the drum, the bean temperature starts rising gradually. Honestly, I consider the concept of the “turning point” (TP) as a phantom idea. Why? The widely discussed “TP” is mostly the result of how your temperature probe records the bean’s temperature. When you charge your green beans into the roasting machine, an immediate decline in temperature is recorded as the cool beans encounter the preheated sensor of your bean probe.
Around 100 degrees C (212 degrees F), the roasting cycle really takes off. This is when the free water content of the coffee passes the boiling point of water; steam develops and now the bean’s volume and internal pressure starts to increase. The coffee loses mass and density, and we can perceive a gradual color change from green to yellow.
As a rule of thumb, the drying phase should last between 35% to 45% of the overall duration of the roasting process. If the drying phase evolves too fast, it will result in an uneven heat distribution inside the bean with an increased risk of internal and external scorching.
Maillard reactions
Next are the Maillard Reactions. Louis Camille Maillard (February 4, 1878 – May 12, 1936) was a French physician and chemist. In 1912, he started studying the reactions between amino acids and sugars. As a result of his contributions, the Maillard reactions were named after him. Basically, the Maillard reactions are also known as the “browning phase”. This is when you can notice the gradual color change of the coffee beans being roasted. If you would like to properly comprehend the Maillard reactions, then I recommend that you brush up on your chemistry knowledge. They involve a series of reactions between sugars and proteins or amino acids, escalated by heat and leading to dramatic chemical changes of the coffee. Numerous coffee flavor compounds are formed because of the Maillard reactions, including the coffee aroma and flavor compound, 2-furfurylthiol.The major Maillard reactions occur approximately between 150 C and 200 C (300 F to 392 F).
Caramelization
Ok, are you still with me?
Let’s continue to review the third stage of chemical transformations: Caramelization. This phase leads to the breakdown and transformation of various types of sugars inside the molecular structure of the coffee beans. When the beans start changing color, from yellow to brownish, that’s when the caramelization takes off and we gradually approach the first crack and the development phase.
The first crack is basically the consequence of the coffee beans expanding rapidly because of increased steam pressure. The cracking or popping sound is created while the external layers of the beans separate from the center cut. At the start of the first crack, that’s when we start timing the actual development phase. Countless chemical reactions continue to evolve. The rate at which they occur is largely defined by the kinetic energy created earlier in the roast.
Along with the coffee’s final bean temperature, the development phase will allow you to modulate the sweetness and perceived acidity of the coffee. For filter coffee, I recommend maintaining a duration of 12% to 18% of the overall roasting time for the development phase and up to 22% for espresso roasted beans. A shorter development phase will express the acidity of the coffee in a more pronounced way. A longer development will create more caramelized notes with lower acidity.
Endothermic versus exothermic heat
Additionally, I’d like to comment on the concepts of endothermic versus exothermic heat. During the endothermic phase coffee beans absorb energy from the roasting machine. The exothermic phase features a reaction that allows heat to be released from the coffee. The coffee beans are endothermic (endo: “in”) for a major part of the roasting process from the start of the roasting process up to the first crack. Right before the start of this first crack, the coffee enters a relatively brief exothermic (exo: “out”) phase. Between the first crack and the second crack the coffee goes through another endothermic phase, which is followed by a final exothermic phase during which the coffee enters the second crack.
Willem Boot
Willem Boot is Brand Ambassador of Giesen Coffee Roasters and founder of Boot Coffee Campus, a leading coffee training institute for the coffee industry. Check the program of specialized courses at www.bootcoffee.com.
Want to know more? Boot Coffee offers various classes.






